Table Of ContentVawne 28 (2010)3055-3065
Contents lists available at ScienceDirect
Vaccine
ELSEVIER j o urn a I home page: www .elsevier. co m/1o cate/vaccine
Vaxfectin® enhances both antibody and in vitro T cell responses to each
component of a 5-gene Plasmodium falciparum plasmid DNA vaccine mixture
administered at low doses
Martha Sedegaha.~. William 0. Rogers a, 1, Maria Belmonte a. Arne! Belmonte a.
Glenna Banania a, Noelle B. Patterson a, Denis Rusalovb, Marilyn Ferrari b.2,
Thomas L. Richiea. Denise L. Doolana.3
• Molaria I'Togram. Naval Medical Research Center. 503 Robert Grunt Avenue. Silver Spring, MD 20910-7500. USA
0 Vicullnc. . I 0390 l'ocijic Center Courr. San Diego. CA 92121·4340. USA
ART I C LE I N FO ABSTRACT
Article lilstory: We previously reported the capacity of the cationic lipid-based formulation. Vaxfectin~. to enhance the
Received 24 july 2009 immunogcnicity and protective efficacy of a low dose plasmid DNA vaccine against Plasmodium yoelii
Received in revised form 8 October 2009 malaria in mice. Here. we have extended this finding to human Plasmodium falciparum genes. evaluating
Accepced 12 OCiober 2009
the immune enhancing effect ofVaxfectin"' forrnularion on a mixture. designated CSLAM. of five plasmid
Available online 30 October 2009
DNA vacdncs encoding antigens from the sporozoite (Pj(SP. l'f.>SP2(fRAP). intrahepatic (l'jlSA 1 ). and
erythrocytic (/'JAM A1 , I'/MSP1) life cycle stages of 1'. fulcipurum administered at 2. 10 or 50 J.Lg doses.
KMe.1y1waroiard s: Vaxfectin"' formulation enhanced both antibody and cellular immune responses 10 each component of
the multi-antigen vaccine mixture. as assessed by EUSA,IFAT, and IFN--y Ells pot. respectively. There was
Trells
Mulci-genc no apparent antigenic competition. as indicated by comparison of responses induced in mice immunized
Plasmid DNA vaccines with P}CSP vs. CSLAM. These data showing that Vaxfectin.., can enhance the immunogenicity of plasmid
Immune enhancement DNA vaccines administered at low doses per body wcighr. and in combinations. has important clinical
implications for the development of a vaccine against malaria. as well as against other public health
threats.
Published by Elsevier Lid.
1. Introduction halted at the liver stage. can protect against subsequent sporozoite
challenge. Protection is believed to be mediated by cell-mediated
In humans and animals. immunization with Plasmodium spore immune responses directed against multiple proteins expressed by
zaires attenuated by radiation. such that their development is irradiated sporozoites after entry and limited development in hep
atocytes [1.21. Naturally acquired clinical immunity. observed in
residents of malaria endemic areas who have experienced repeated
malaria infections. is effective at preventing severe malaria: and
AbiJreviarions: Ai'Cs. antigen presenting cells: Cl, confidence interv.11: D-D. is thought to be mediated by antibodies directed against multiple
DNA-DNA immunizalion regimen: D-V. DNA-recombinanl vir.ll boos! immuniza antigens expressed in the blood stage [3.41. These models establish
lion regimen: ELISA, enzyme-linked immunosorbenl assay: ELISpol. enzyme-linked the feasibility of developing a malaria vaccine but. despite extensive
immunospol: IFA . immunonuorescent antibody 1es1: IFN--y. incerferon-gamma:
research spanning decades. an efficacious malaria vaccine is not yet
pDNA, plasmid DNA: P/. Plasmodium falcipanun: CSP. circumsporozoite prolein:
SSP2. sporozoile surface procein 2: l5A1. liver scage ancigen-1: AMA1, apical available [5-7j. The level of protection obtained to date with sub
merozoite ancigen-1: MSP 1.2 IJD7 strain). merozoite surface proccin-1: SFC. spot unit vaccines based on a single antigen has been suboptimai[S-131.
forming cells. and there is increasing recognition of the need for vaccines target
• Corresponding author ar: Malaria Program. U.S. Milicary Malaria Vaccine Pro ing more than one antigen and more than one stage of the parasite
gram. Naval Medic.ll Research Center Component, 503 Robert Grant Avenue. Silver
life cycle, and Lhat induce both cellular and humoral immunity.
SprinEg-m. MaiDl a 2d0dr9e1s0s-:7 M50a0rt.h UaS.SAe.d Teegl.a:h ~@1 m30e1d .n3a1v9y 7.m58il6 (:M fa.x S: e~dtc g3a0h1). 3 19 7545. Accordingly, the research focus has moved towards development of
1 Current address: Naval Medical Research Unic2. Americ.ln Embassy jakarta, Unit a multi-stage multi-immune response vaccine comprising antigens
8132. FPO AP 96520-8132. Indonesia. expressed in the liver stage and targeted by T-cell responses as well
2 Currently: Independent Consultant. as antigens expressed in the blood-stage and targeted by antibody
3 Current address: The Queensland Institute of Medical Research. The Bancroft
Cemrc. 300 Herston Road. P.O. Royal Brisbane Hospit,JI, Brisbane, QLD 4029, Aus responses [141. By reducing the numbers of parasites emerging
tralia. from the liver(T-cell immune responses directed against liver stage
02G4-410X/S-see front mauer. Published lly Elsevier Lid.
dol: 10.10 It i/j.vacclne.2009.10 .044
Report Documentation Page Form Approved
OMB No. 0704-0188
Public reporting burden for the collection of information is estimated to average 1 hour per response, including the time for reviewing instructions, searching existing data sources, gathering and
maintaining the data needed, and completing and reviewing the collection of information. Send comments regarding this burden estimate or any other aspect of this collection of information,
including suggestions for reducing this burden, to Washington Headquarters Services, Directorate for Information Operations and Reports, 1215 Jefferson Davis Highway, Suite 1204, Arlington
VA 22202-4302. Respondents should be aware that notwithstanding any other provision of law, no person shall be subject to a penalty for failing to comply with a collection of information if it
does not display a currently valid OMB control number.
1. REPORT DATE 3. DATES COVERED
OCT 2009 2. REPORT TYPE 00-00-2009 to 00-00-2009
4. TITLE AND SUBTITLE 5a. CONTRACT NUMBER
Vaxfectin enhances both antibody and in vitro T cell responses to each
5b. GRANT NUMBER
component of a 5-gene Plasmodium falciparurn plasmid DNA vaccine
mixture administered at low doses 5c. PROGRAM ELEMENT NUMBER
6. AUTHOR(S) 5d. PROJECT NUMBER
5e. TASK NUMBER
5f. WORK UNIT NUMBER
7. PERFORMING ORGANIZATION NAME(S) AND ADDRESS(ES) 8. PERFORMING ORGANIZATION
Naval Medical Research Center,Malaria Program,Silver REPORT NUMBER
Spring,MD,20910
9. SPONSORING/MONITORING AGENCY NAME(S) AND ADDRESS(ES) 10. SPONSOR/MONITOR’S ACRONYM(S)
11. SPONSOR/MONITOR’S REPORT
NUMBER(S)
12. DISTRIBUTION/AVAILABILITY STATEMENT
Approved for public release; distribution unlimited
13. SUPPLEMENTARY NOTES
14. ABSTRACT
We previously reported the capacity of the cationic lipid-based formulation. Vaxfec tin"’. to enhance the
immunogcniciry and protective efficacy of a low dose plasmid DNA vaccine against Plasmodium yoelii
malaria in mice. Here. we have extended this finding to human Plasmodiumfalciparum genes. evalua ting
the immune en hancing effect ofVaxfectin"’ formulation on a mixture. designated CSLAM. of five plasmid
DNA vaccines encoding antigens from the sporozoi te (Pj(SP. Pj5SP2{fRAP). intrahepatic (PjlSA 1 ). and
erythrocytic (1’/AMA 1. I~SP1 ) life cycle stages of 1’. falcipurum administered at 2. I 0 or 50 1-1-g doses.
Vaxfec tin"’ formulation enhanced both antibody and ce ll ular immune responses ro each component of
the multi-an tigen vaccine mixture. as assessed by ELISA, IFAT, and IFN-"( ELl spot. respectively. There
was no apparent antigenic competition. as indicated by comparison of responses induced in mice
immunized with P}t:SP vs. CSLAM. These data showing that Vaxfectin "’ can enhance the
immunogeniciry of plasmid DNA vaccines administered at low doses per body weigh t, and in
combinations. has important clin ical implications for the development of a vaccine against malaria. as well
as against other public health threats.
15. SUBJECT TERMS
16. SECURITY CLASSIFICATION OF: 17. LIMITATION OF 18. NUMBER 19a. NAME OF
ABSTRACT OF PAGES RESPONSIBLE PERSON
a. REPORT b. ABSTRACT c. THIS PAGE Same as 11
unclassified unclassified unclassified Report (SAR)
Standard Form 298 (Rev. 8-98)
Prescribed by ANSI Std Z39-18
3056 M. Sedegah et al./ Vacdne 28 (20 10) 3055-3065
antigens) and priming the immune system to antigens that will be dose PyCSP pDNA formulated in Vaxfectin® enhanced levels of
boosted by infection from natural exposure (antibody responses antibodies, IFN-')' responses, and sterile protection after chal
directed against blood stage antigens), it is hypothesized that one lenge [29]. In other studies in mice, rabbits, and other animals,
will reduce the severity and mortality due to Plasmodiumfalciparum the use of Vaxfectin® formulation to deliver vaccine antigens for
malaria. Other research is being directed towards the develop anthrax and influenza induced strong systemic, long-lived, and
ment of whole organism vaccines such as the irradiated sporozoite antigen-specific antibody responses [30-36,38,39). Vaxfectin®
vaccine being commercialized by Sana ria Inc. [15) and genetically formulated influenza H5 hemagglutinin-containing pDNA vaccines
attenuated parasites [16-19) which rely on the expression of the have undergone Phase I testing in humans with encouraging results
entire repertoire of parasite antigens expressed in given stage of (37). Vaxfectin® was also shown to enhance antibody and T-cell
the parasite life cycle. It is well established that antigen variability responses to a protein-based influenza vaccine in mice [40).
is a major obstacle to malaria vaccine development [20,21). The In the current study, we hypothesized that. when tested at
immunological challenges to developing effective malaria inter the same pDNA dose, the breadth of the total anti-malarial T
ventions have been recently reviewed (22). cell response induced to a 5-gene vaccine, CSlAM, will exceed the
Based on the two models of human immunity against malaria response induced to a single gene vaccine. Our second hypothe
irradiated sporozoite immunization and naturally acquired immu sis was that a homologous D-D immunization regimen with low
nity - we have sought to develop a DNA-based malaria vaccine dose pDNA, involving single antigen or with a 5-gene vaccine for
which relies upon the induction of antibody and T-cell responses mulated with Vaxfectin® will yield immune responses comparable
against multiple parasite proteins expressed in different stages of to those obtained with higher dose pDNA of the same vaccines
the parasite life cycle. Our previous studies of mice immunized given without formulation. In general, priming with pDNA followed
with a mixture of plasmid DNA (pDNA) encoding nine P. falci by boosting with recombinant vaccinia virus (D-V) dramatically
parum (3D7 strain) antigens revealed suppressive effects in the increases the immune response as compared to responses induced
multi-antigen mixture which could be associated with a specific by D-D regimens [41,42]. Therefore, our third hypothesis was that
subset of the nine antigens (23). Those data led us to down-select aD-D immunization regimen with low dose pDNA involving single
a core panel of five antigens, designated CSlAM ( CSP, SSP2, LSA 1, antigen or with a 5-gene vaccine formulated with Vaxfectin® will
AMA 1, and MSP1 ), for further development and evaluation. The yield enhanced immune responses comparable to or approaching
pre-erythrocytic stage antigens (CSP, SSP2, and LSA 1) are designed those obtained with unformulated low dose pDNA administered
to induce T- cell responses against liver stage antigens, and the in a heterologous DNA prime-viral boost (D-V) immunization reg
erythrocytic stage antigens (AMA 1 and MSP1) are aimed at induc imen.
ing responses directed against blood stage parasites. Since AMA 1
and MSP1 are also expressed in sporozoites and liver stage par 2. Materials and methods
asites [24-261 they are expected to also contribute to hepatic
stage immunity. The underlying rationale is that, upon vaccina 2.1. Mice
tion, the combination of responses from the pre-erythrocytic and
erythrocytic stage components could result in sterile protection Six to 8-week-old female inbred BALB/cByJ (H-2d) mice were
by eliminating all or most of the parasites developing in the liver purchased from The jackson Laboratory (Bar Harbor, ME) and used
(pre-erythrocytic immunity) together with a backup immunity that for cellular response studies. Six to 8-week-old female outbred CD-
would limit and clear any breakthrough parasites that evaded the 1 mice obtained from Charles River Laboratories (Wilmington, MA)
liver stage immunity to develop blood stage infections (erythro were used for antibody response studies.
cytic immunity).
One limitation of multi-antigen vaccines is the amount of pDNA
2.2. Plasmids
injected and the potential need for a highly concentrated pDNA
mixture which could affect the efficiency of transcription and trans
Plasmid DNA (pDNA) stocks encoding each of five P. falci
lation and thereby result in a suppressed or suboptimal immune
parum antigens (307 strain), CSP, SSP2, LSA 1, AMA 1, and MSP1,
response against target antigens. Moreover, the modest immuno
as well as empty vector without insert, VR1020, were produced
genicity of some pDNA vaccines to date in human and nonhuman
by PureSyn, Inc. (Malvern, PA). All plasmids have been previ
primate studies that involved needle injections may be attributed,
ously described [23). All pDNA stocks were ~90% supercoiled.
at least in part, to the relatively low dose per body weight as com
Vaxfectin®-formulated pDNA stock endotoxin levels were less than
pared to murine studies. The upper limit of total pDNA that can
30 EU/mg, while the unformulated pDNA stock endotoxin levels
be injected is influenced by both the concentration and viscosity
were less than 7.5 EU/mg.
of the vaccine and the injection volume. Finally, the manufactur
ing costs associated with high dose and requirements to include
2.3. P.falciparum recombinant virus (NWAC-PP)
multiple antigens of interest for some pathogens, detract from the
often-cited advantages of pDNA vaccines.
The recombinant attenuated vaccinia virus. NYVAC-Pj7. used in
In both animal and human studies that involved needle injec
boosting experiments has been previously described (23,43,44 ].
tions, the immune responses induced by DNA prime followed
NYVAC-Pj7 expresses seven P. falciparum antigens comprising Pf
by DNA boost (D-D) immunization regimens often follow a dose
CSP, Pf SSP2, Pf LSA 1, Pf SERA, Pf MSP1, Pf AMA 1, and Pf Pfs25. The
response [27,28). This implies that in order to induce optimal
Pf CSP, Pf SSP2, Pf AMA 1, and Pf Pfs25 antigens derive from the
responses against all antigenic components in an optimal pDNA
NF54/3D7 clone of P. faldparum, the Pf l.SA 1 antigen derives from
mixture without significant interference from some specific plas
the NF54 strain of P.falciparum, and PjMSP1 and SERA derive from
mids, one may still have to meet a certain dose threshold for
the Uganda-Palo Alto and FCR3 strains, respectively.
all individual pDNA components in the mixture. To avoid possi
ble suppressive effects due to the use of high dose pDNA and to
reduce cost. we embarked on a series of studies which capital 2.4. Vaxfectin® formulations
ized on the ability to formulate low dose pDNA with Vaxfectin®
for induction of robust antibody and T-cell responses. We have PlasmidfVaxfectin® formulations were prepared as previously
previously shown that, compared to PBS-formulated pDNA, low described [29]. Briefly, Vaxfectin® in sterile water for injection
M. Sedegah er al./ Vaccine 28 (2010) 3055-3065 3057
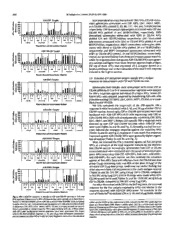
Experiment I:
t"'l' tSI AM
2 IU. •'if ~···~ ·h JlllNA :1,1CI,Ill ~)Jllof·" III)NA
PBS Yaxfectin® PBS Yaxfectin®
IIC1a(•l,~.,,u,.
J).V
<.:W <.SI,\M
.: Ill N ~lllf :, rf>\tA !. Ill. •lf ~it'F t, rUN\
_____v_ a_x_re.c.-ti-n_®_ __ ,] ..._[_ ___P_ B_S~---' Yaxfectin®
Experiment 2:
llomultlp('\1 ..
I~IJ
t'SI'. S!\112. I ....: .A lo~\~11\ I.MSI11
1, pe:. '' 11I>NA
(~.,. Yaxfectin®
______P_ B_s_ ___ ,] .(..__ _v_ a_x_re_c_ti_n®_ __ ,] ___r_ B_s_ ___ .)
C~P SSI~ ISAJ \M \l.~t.'il'l (SIJ\M
1 tip 2\rDS\ : I•F ,:, rUS-\
1\lh:. .. rfu NYVt\l'·l'li
Experiment 3:
llclemkt~('11\
11-V
t~I'.!"SI':. J SAI.A~It\ I.M~I'I lSI AM
~~~ ,,~ ·" riJ~,, H4JI!L I\ rllNt\
- l
PBS PBS Yaxfectin®
Fig. 1. Fluxogram of immunizations. All injections were given at 4-week intervals and samples for antibody and/or lFN--y EllS pot responses were assayed at 2 weeks after
last injection.
(SWFI) was added to an equal volume of pDNA (single plasmid or inson 309301) with 29G1/2 needles. Individual plasmids or the
plasmid mixture) at twice the desired final concentration in 2 x PBS CSLAM combination were administered at the same total pDNA
(20 mM sodium phosphate, pH 7.2, i .8%N aCI). Formulations were dose and in the same number of sites: therefore, for each antigen,
mixed by gentle inversion. The final molar ratio of pDNA:cationic the actual amount of plasmid in the 5-gene-CSLAM plasmid mix
lipid was 4:1. ture was always one-fifth as compared to the individual plasmid
vaccine.
2.5. Immunizations In Experiment 1, three pDNA doses that included 2, 10 and
50 fJ.g of CSP and CSLAM pDNA vaccines in PBS or formulated in
Mice (n=14 per group for antibody studies in CD-1 mice and Vaxfectin® were tested in either homologous D-D or heterologous
n = 8 per group forT-cell studies in BALB/c mice) received intramus D-Y immunization regimens (D-D and D-V immunized in parallel).
cular injections in the tibialis anterior muscle pDNA formulated The homologous immunization regimen groups received 4 doses of
in PBS or Vaxfectin® using insulin syringes (0.3 ml: Becton Dick- 2, 10 or 50 fJ.g pDNA in PBS or formulated in Vaxfectin®, whereas
3058 M. Sedegoh era/. I Vaccine 28 (2010) 3055-3065
the heterologous immunization groups received 3 doses of 2. 10 same manner. Due to the large number of groups of mtce involved.
or 50 JJ.g pDNA in PBS or Vaxfectin® followed by an intramuscular pooled splenocytes were used in some experiments (Experiments
boost with 1 x 107 pfu NYVAC-P[l recombinant virus. Three con 1 and 2). For the pooled cell assays, assessments were done at least
trol groups included mice injected 3 times with empty vector pDNA twice using previously frozen cells. The data presented herein for
formulated in PBS or Vaxfectin® and boosted with NYVAC-P[l, and Experiment 1 utilized frozen splenocyres. and Experiments 2 and
naive mice. Injections were carried out at 4-week intervals and 3 utilized fresh splenocytes for the ELISpot assays. We had previ
samples for antibody and IFN--y ELISpot responses were assayed ously compared the results of fresh or frozen cells assays and found
at 2 weeks after the last injection. that the magnitude and pattern of responses were similar(Sedegah.
In Experiment 2, each of the five plasm ids included in the CSLAM unpublished).
vaccine was injected individually at a dose of 2 JJ.g per mouse. Seven APCs were prepared by transfecting A20 cells with plas
CSLAM vaccine was also injected at a dose of 2 JJ.g per mouse. mid encoding CSP, SSP2, LSA 1, AMA 1. MSP1, CSLAM mixture, or
After 2 priming injections with pDNA, the homologous immuniza unmodified plasmid VRl 020, using the AM AXA nucleofector sys
tion regimen groups received a third pDNA immunization. while tem (AMAXA Biosystems{Lonza Colgone AG. Basel. Switzerland)
the heterologous immunization groups received an intramuscular according to manufacturer's instructions. Eighteen to 24 h after
boost with 1 " 107 pfu NYVAC-P[l recombinantvirus.IFN--y ELISpot transfection. transfecred cells were washed three times with com
responses were assessed in pooled splenocytes from each group 2 plete medium, suspended in 2 ml complete medium. irradiated at
weeks after the last injection. 10K rads, counted. and used as APCs in the ELISpot assay [23].
In Experiment), each of the five plasm ids included in the CSLAM Splenocytes were stimulated in vitro with different A20-transfected
vaccine was tested at an even lower dose of 0.4 JJ.g per mouse. After APCs and the number of Pf antigen-specific IFN--y-secreting spot
a single pDNA priming. all primed animals received an intramuscu forming cells was evaluated after a 36-h culture period. Splenocytes
lar boost with 1 x 107 pfu NYVAC-P[l recombinant virus. Another were tested at 400K. 200K. and 1 OOK per well while APCs cells were
group of mice received no pDNA priming, but an intramuscu tested at 1 OOK per well. Assays were done in triplicate and the num
lar injection with 1 x 107 pfu NYVAC-P[l recombinant virus. IFN--y ber of IFN--y-secreting cells. recognized as spot-forming cells (SFC)
ELISpot responses were assessed in individual mouse splenocytes was counted using an automated EllS pot Reader manufactured by
2 weeks after the last injection. Autolmmun Diagnostika (AID) (GmbH, Strassberg. Germany). The
A nuxogram of all three experiments is illustrated in Fig. 1. number of antigen-specific IFN--y SFC in test wells was corrected for
background by subtracting the mean number ofSFC of the negative
2.6. Antibody U$SDYS control wells (A20 cells rransfected with empty plasmid VR 10 20)
and the mean of the test sample was calculated. Data were pre
Individual sera obtained 2 weeks after the last injection (Experi sented as the number of IFN--y-secreting SFC per million spleen
ment 1) were analyzed for reactivity with P.falciparum sporozoites cells.
and blood stage parasites by IFA as previously described [8]. Sera
were also analyzed by standard ELISA as previously described (45]. 2.8. Swristicul una lysis
The expression. purification, and characterization of the five P.fa/
ciparum recombinant proteins used as capture antigens. CSP, SSP2. IFA and ELISA titers generated from Experiment 1 were log
LSA 1. AMA 1. and MSP1 has been previously reported [23]. transformed for analysis. and all calculations of confidence inter
vals were carried out with log transformed data. The mean ofth e log
2.7. IFN-y EL/Spot assays of the titer in each group and the difference in the mean log titer
between corresponding Vaxfectin® and PBS groups are reported.
Detection of antigen-specific IFN--y producing cells was per Positive log titer differences where the 95%c onfidence interval (CI)
formed as previously described [23] with modification. Since H-2d does not involve a 0 or a negative number represent a significant
restricted T cell epitopes have not been defined for all antigens difference. Statistical analysis of the anribody data was performed
studied here. and because responses could be directed at multiple with Stata/SE version 8.0.
epitopes on a given antigen. we used transfected A20 cells (ATCC) For rhe IFN--y EUSpot data analysis in Experiment 3. data gen
as antigen presenting cells (APCs) in the ELISpot assay. For the erated from individual mice (8 mice/group) immunized with the
5 antigens, this approach offered an efficient and more practical D-V regimen were analyzed in a 2-way analysis of variance. con
means of measuring T-cell responses in all experimental groups ducted with log transformed or untransformed data for three data
simultaneously against all five antigenic targets prepared in the sets. namely (1) the individual vaccines tested against the individ-
Tdble 1
IFA response in CD-I mice to l'.fulciporum vaccines given by D-D and D-V regimens (Experimem 1 ).
Regimen Dose (Jl.Jl) CSP vaccine CSI.AM vaccine
Sporozoite IFA Sporozoi1e IFA Blood Stage IFA
VAX PBS Difference log VAX PBS Difference log VAX PBS Difference log
titer (95% Cl) titer (95% Cl) titer (95% Cl)
D-D 2 5,653 2.826 545 552 4.064 1452 0.45 ( -0.06. 0.96)
10 9,275 7.994 2,436 1.159 1,689 2560 -0.18 ( -0.80. 0.44)
50 16,800 4.873 2,970 4.363 1.140 3121 -0.44 (- 1.12, 0.25)
D-V 2 17.653 9,275 11,880 3.121 0.5& (0.20, 0.96) 5.120 2970 0.24 (-0.26. 0.73)
10 33.601 12.058 21,519 7,994 0.43 (0.16, 0.70) 10,240 4413 0.37 ( -0.02, 0.75)
50 19,491 24,965 15,217 13.116 0.06 (-0.23, 0.36) 2.970 8400 -0.45 (-1.12, 0.22)
Sera from individual mice (n•14) were assayed ami geornerric mean endpoim tilers for each group against f'.jalciporum sporozoires or blood sr.tges hy IFA are repo11ed. To
compare the effect of Vaxfectin« formulation on ami body response. the individual endpoim antibody liters were log transformed and !he difference on the mean log titers
between corresponding Vaxfectin~ and PBS groups and the 95% confidence interval (CI) around the difference were obtained and arc reponed in the .. Difference log titer
(95% Cl)" column. Positive log titer differences where the Cl docs nor involve a negative number or 0 represent significant difference (highlighted).
M. Sedegoh er aLI Vaccine 28 (20 10) 3055-3065 3059
ANAultehPPaCveClCSe ASssTl ., 2 uosaP0kfon a eAfsdityPri wg(w-C3naKis)sir.f r etei(ach 2a(mec)Nn o CetCchmrSeS eLp.Sm Aia,Sn uM2rtdila0 stivtv0oiiapisn7dclts)ecui. c ioaanclfole vtm aaahngpceaaca lgiiyrnnrsiessoisotsu in tpnwe dsmpaitrsveeo idapdc neuaesdagr fualwo airnreenms rdteae AA td m22 tu00ha--sedCCi enSS( OgwLL .AOAtihtMMShe) --,o"st~~:, :·":eu}:E"~<:' '- "-"".0.'-...1II..'... '" ..""'-.00".:1.''II.'_' " :1N"0I: o~oC"q~I-' l. ,Nov"""~0~;.:!'\-:C",:'"0-!!f-_:=!'IiI 'oI".I"(0.-ro';"'~:,I)ii' .<'t-.:g".c0cEi!.l) ,: ~-·"~.~,-c~~:0..:..
""c.''. -~_"."N',',"M.".',' .'c'..".c..".~. .gN.. , .~: -oE"c: ....:..
3. Results 1;; ~ ."",''""_''"."",' ."eo,n'>.. ,,No...,Cr,-.C-, ~tx-"u='.-"=~
:;[ > N g~~
3.1. Effect of Vaxfecrin® formulation on porasice-specijic or _o_ 0.~§"' ~:·-;
oncigen-specific oncibody responses induced by immunization cOof'o!Nn ~~§' M-
with CSLAM and CSP vaccines -~::u:- "OM....':v"Nli'.'.d".r..::' -c:=go(cioo~ .c.O.:.J Qu.c.J:. ".'c.-.:'.
SO fO.Lgu tdborseeds C oDf -C1S mP iocer (CnS =L A1M4/ gprDoNupA) v waecrcein iems minu nPiBzSe do rw Vitahx f2e, c1t0in o®r -i~c~:~S. ,e·}E..~.~.. ."o".I! .'".."...!'I'.: ."".o.!I''. . "0JW.'- .10.c!"Ic. . !.0".....!I!. , -CoEO~J".'"icn0-'8: =-u~~~-o
ivatznioinoa itnS tighe ewseoc nmweti-roeosenplr o eeag 2scho.si ifuagiScyshee ear0danr- Dtifinocb o room lrdlpe iiachcereetsae t sibedmi rytoem 2E-l osuLwgpInSoeeiAzuce.eisk fdI isFc0 Aw-aaV finatt nhetrietrb iC gobStidomhPdieee py sn lD astbiN,st yetAa rsI siF am dAltoeom nas suecnp rndtioih bzrfaeaoond--r ::<;;;[: ""<VX>-' I """,M~"-....''"' """..N"...'..''.. '".""N.~."...''''.. .(".l..__..,ll....'i:....,.O...af.....,2..,:...i,, , ~N.l;....Col2.,,ni. \ .-.t.o"U"c0~~.:..c :.''. . C c ..4':"I~."cc=;\?J..aU.. ' : ":··c:".~~~.0:-;.':;.0'
in mice that received the CSLAM pDNA mixture (Table 1 ). How- "'=V'IO
<CO~
mieranevtstuteaoplrit .ena i-ctechatcdiinovs tewu idglnyhietde) n. ( n n2cA o tosaht mn erede bxa nipc1neet0aucc tfta.tiL oelsg dnia g.C msnSmiinPofi ciucceeneaq ct nu iootimv mfsa mCuplSpeaupPnrnart eii bnztselo esdt ih1va ee0wn tCaeiitbnSfhfodLe Ad cCSMytSOs l L edfwA.LvuaMgee sl C s thSt owaaL kdeAthe rMenea -~tc~~::u:t·e."=o:e-' .0DdcId-.!D.i.;do0.C'.d§!Cv.< .'0.oVdM.g!f. .' 2-ac•IUO-D)D~cfq c."oN"M.oc'o:il. 'o~"o~o"l.Bl', .·wV~~'C-0~~o-l-. !: !:."'"!=:>\u'c);t-;.:(: l: !·="CcE0:>~~ ~::)'
awgiadien sbtr ebalodothd ostfa rgeessp oans swe ealsl iansd sicpaotreodz obiyt eds e(tTeacbtaleb lIe) I FaAn dr ebsyp oEnLsISeAs "iVc:S'.I "'"'"' N_t.D,.~, :"c:5 ·~-§~~c:
against each of the CSLAM proteins (Table 2). Formulation ofCSLAM < <X coco~ )e!::~ E0 "c'. ~0
banodth C ISFPA vaancdc iEnLeIsS Aw tiitthe rVs.a xrefaeccthiinn®g gsetanteisraticllayl lseidg ntoif icaann icnec riena ssoem ine ~ > ~~- 0N Eeu~ "~&u') .:>oc::
cases (Tables 1 and 2). The immune enhancement conferred by -~=u "d".c ,.:.,·i 0~. .8. ~ ~
Vaxfectin® formulation was generally more pronounced at the low- tc:o.ore. "d1o11V')0! .._s9! ..-.~ 'c0;
esusct hd oasse l otews tdeods e(2 p fD.LgN) Aa nadn di nh soumboolpotgiomuasl 0im-Dm ruanthizeart itohna nr ehgeitmeeronls- !c~"'E·~=~~ d~NCd~C..d!i.n- 0.c.,.. ::=-~0".-:1:':
ofignoduinsg 0s- Vin rtehgei mPelanssm (oTdaibulmes y 1o ealniid C 2S)P, cmonosdiesltel2n9t w]. iNthe xotu, rw per eevviaoluus- ...., "g': """'"M' I_l.l,"-".'."',c-.o' ·;ec~~:; : .-=eo~:= i"~~'
aretesdu ltw ihne athnetirb ofodrym luelvaetlisn gco lmowpa droasbele p tDoN tAho wsei thac Vhaiexvfeedc tbiny® h cigohueldr .s&t ) VV!:>:i' ~> .,.M._V0'> CO,O.., I_n .e;.;c.:C.I.;C\:1;.1~~~~
pDNA doses without formulation. Data showed that this was gener- wcX . -~~ 4~,1"·-'·"-
aipnlDl yVN taAhx eifen cc aPtsiBneS ® s(i Tnwaceebr leree sss i1pm oainnlasder 2st )oi.n Itdhnuo tcsheeed ic nbadysue 2 co ef.fLd gC bCSySL PAS MpOD f.hLNgoA wd ofeosvreemsr .u oElfaL CItSeSAPd ."E"c~.':'. -~:C~:; :uV=e)tl 0c~~~ c~\c-.!.i.g.0.~d! .. ·~-i-~:g-::::! _ ~'..~rc~:;0u:: ··=~~~--
antibody responses induced against two of the S antigens, AMA 1 > ~~ d~d -~
aonf d2 fM.LgS PClS LbAyM V paxDfNecAt info®r mwuelraet endo itn i nVcarxeafescetdin e®x cine ptth ein D t-hVe rceagsie- '00"c0 :' '.8"c.:' VO.',o ·C :";'".~....,"o:.v_m"-) ,.~...g.:,N!_I :V.,:'.\ 'cQc00:3:J: "E.,c-.0;:.) Q50-c. :,
men (Table 2). We also asked whether Vaxfectin® formulation of 0, "' E~O
opDf aNnAti bwooduyld r easllpoowns teh ea phpormoaoclohginogu st hDo-sDe raecghimievean btloe awchitihe vteh ele hveelts- ...>c0..:.. ::"u>5; '[ ecJ.. <>X .N","V,_"£'M",a..'i, ""N_".',""O."l."".-,'l ',-.EoCuO._ J"o.";C_;.'~; .. ,' ..~nU_-..,...
erologous 0-V regimen. Our results showed that while Vaxfectin®
formulation enhanced antibody levels at each of the three doses ·;""c::;'' .. 'g0 ~~ -~~
(teTsatbeldes, le1v aenlsd d2i)d. not surpass those obtained by D-V immunization E"">'' -to u:.=. ""~C' ~-O=tJ-. ;Q0; .
2 ""' ~~~
0 ~e ... 0 ~
.!..;.!.- ~t ~-g~
3.2. Use of multi-antigen antigen presenling cells (APCs) 10 :E. a·= -~ rc.2: ·P-
measure P. falciparum ancigen-specijic IFN-y ELlSp oe responses o.; """'"' fll~~ ~ -~ ~
E ~~~ ec-ce
eSxppleWrneeso scciyantrger site hfdre oo mSu atm na itcpigeree inlmism msiinumanuriyzlt eaadsns ewaoyiu thtso l yleo viwna ldIuFoaNstee-" (pt hEDeLN IuSAsp e(o2 ot ff.aL Agss,P a0Cys-. ·.u0E="u ' "ce"c>O8l.:J "uc .' ""c>~''. N...........:.,:..;..-,.;..:.;. ,"agM..'i -,aI~~.D.i0_-NM"N1.',OM"Oo~.o.' ·--e"c~~' :: ~ "·'~c0->0" 0 . g·c-G~c'--J:.
Dto traelg dimoseen) )o irn avso alv minigx ttuhree (SC SanLAtigMe n2s f. Lign jteoctatel dd oasse :i n0d.4iv fi.dLuga/plsla (s2m Ji.dl.g) :c0; :: o0"~' c~-:oi NO-O"' N O_.,O.. ·~~e~~ -~g
were stimulated in vitro with different APCs that included A20 cells N ~~ ..E"c:.' ~ ~g ~_E
transfected with plasmid encoding CSP, SSP2, LSA 1, AMA 1, MSP1, :.E,~_, "' 60 6> r: c. ""
<:<: .,~15
or CSLAM mixture. 1-W Vl w 0
3060 M. Sedegah et al./ Vaccine 28 (20 J 0) 3055-3065
In an example of an assay that utilized 1O OK APCs, CSLAM immu
nized splenocytes stimulated with CSP, SSP2, LSA 1, AMA 1, MSP1,
and CSLAM APCs yielded 5, 85, 88, 117, 154, and 316SFC/million
respectively; CSP immunized splenocytes stimulated with CSP and
CSLAM APCs yielded 14 and 20SFC/million, respectively: SSP2
immunized splenocytes stimulated with SSP2 or CSLAM APCs
yielded 124, and 153 SFC/million, respectively; LSA 1 immunized
splenocytes stimulated with LSA 1 or CSLAM APCs yielded 41 and
90SFC/million, respectively; AMA 1 immunized splenocytes stim
Plasmid Dose aad lmmaaiutlon Regimen
ulated with AMA 1 or CSLAM APCs yielded 38 and 78 SFC/million
A2D-CSLAM Targets respectively: and MSP1 immunized splenocytes stimulated with
MSP1 or CSLAM APCs yielded 18 and 16SFC/million, respectively.
Overall, our general finding from these preliminary assays was that,
while the responses detected against A20-CSLAM APCs were gener
ally variable and higher than those detected against single antigen,
the use of these APCs that expressed all 5 antigens served as a
helpful additional tool in the evaluation of the total T- cell response
csr.oo CIJ.U~DD CIIAUDV c ......... induced to the 5-gene vaccine.
Plum lei Dose ll1ld lmmgaization Rqlmea
3.3. Induction ofP.falciparum antigen-specific IFN-y ELISpot
AlD-SSPl Taraets
responses by immunization with CSP and CSIAM vaccines
Splenocytes from BALB/c mice immunized with either CSP or
CSLAM pDNA by D-D or D-V immunization regimens were assayed
for lFN-'Y responses against individual Pf antigen APCs. Seven dif
ferent APCs were assayed, prepared by transfecting A20 cells with
PIIS VAl( PRS VAX Pll'll \'Al plasmid encoding CSP, SSP2, LSA 1, AMA 1, MSP1, CSLAM, or unmod
CSP·PP CSIAUhb CSP.DV <"SIA\1·0\'
ified plasmid VR1020.
Plasmid Dux ~md lmmaaiullon Rqimcn We first compared the magnitude of the CSP-specific IFN-'Y
response in mice immunized with 2, 10, and 50 fJ.g of either CSP or
AlD-LSAI Tareets
CSLAM vaccine. Accordingly, CSP or CSLAM splenocytes were each
incubated with A20-CSP APCs (A20 cells expressing only CSP) or
A20-CSLAM APCs (A20 cells simultaneously expressing CSP, SSP2,
LSA 1, AMA 1, and MSP1 ). Robust CSP-speci fie IFN-'Y responses were
_ detected by both CSP and CSLAM vaccines when A20-CSP APCs
.... were used (Tables 3a and 3b, and Fig. 2). Secondly, each of the vac
PIIS VAX Plllll \'AX P'RS \',U Pill V.U cines induced the strongest response against the matching APCs
CIP.OO C5P-IJ\' C'1iiA\I.IJV
(Tables 3a and 3b, and Fig. 2). In general, it was noted that responses
Plasmid Dose and lmmaaiutioa Rqimen
measured against A20-CSLAM APCs were generally higher regard
less of vaccine (Tables 3a and 3b, and Fig. 2).
Next, we summed the IFN-'Y response against all five antigenic
APCs, as a measure of the total response induced by the multiva
lent CSLAM vaccine. Accordingly, splenocytes from CSP or CSLAM
immunized mice were incubated with the panel of individual anti
genic APCs comprising A20-CSP, A20-SSP2, A20-LSA 1, A20-AMA 1,
and A20-MSP1. For each vaccine, we then summed the responses
PIIS \'.L'I PBS \'.U PIS V.U I'R!I \'41
csr.JlD CINW against all five APCs. Data with effectors from the CSLAM low dose
Plum lei Dox and lmmaai&atlon Regimen group (2 fJ.g) containing only one-fifth of the dose (0.4 fJ.g) of the
univalent CSP 2 fJ.g dose group, confirmed our earlier finding that
A2D-MSPI Tarzets
there was no evidence of suppression in the multi-antigen mixture
(Tables 3a and 3b; 279 SFC using 0.4 Jig CSP in CSLAM, compared
to 582 SFC using 2 fJ.g CSP). Similar findings were made when A20-
CSLAM targets were used (Tables 3a and 3b; 1679 SFC using 0.4 fJ.g
CSP in CSLAM, compared to 598 SFC using 2 fJ.g CSP).
For mice immunized with CSPvia the 0-D regimen, the summed
response for the five antigen-expressing APCs was similar to the
response obtained with A20-CSP APCs alone. For example, in the
Plasmid Dose aad lmmunWitioa Regtmcaa case ofVaxfectin® formulated CSP at 2, 10, and 50 tJ.g doses, results
Fig. 2. IFN-y EUSPOT response in BALB/c to CSP and CSI..AM vaccines in D-D and
D-V regimens (Experiment 1) .IFN-y ELISpot assays were carried out as described in
Section 2. Freshly isolated spleen cells were pooled from 8 BALB/c mice per group pDNA vaccine PfCSP or the multivalent pDNA mixture CSLAM DNA alone regimen
2 weeks after the last immunization and incubated with A20 cells transfected with given as 4 homologous DNA doses 4 weeks apart (DO) or a prime-boost regimen
P.fa/dparum CSP, SSP2, LSAl, AMAl, MSPl, CSLAM, or empty plasmid as control. given as 3 homologous DNA doses followed by a NYV AC-Pn boost dose each 4 weeks
Data is presented as antigen-specific IFN-'Y spot-forming cells per million spleen apart (DV). Control groups were given the DV regimen (3 doses empty pDNA, then
cells (SFC) after background control responses have been subtracted. The X-axis 1 NYVAC-Pn boost) with pDNA in PBS (cV), or with pDNA in Vaxfectin (c vaxV). A
reflectS the immunization regimen: 2, 10, and SO J.Lg total pDNA/dose administra third control group was naive. Error bars reflect standard deviation of quadruplicate
tion formulated in either PBS or Vaxfectin (Vax). Regimens were either the univalent samples.
M. Sedegah ec aLl Vaccine 28 (2010) 3055-3065 3061
Tablela
IFN--y EUSpot response in BAI.B/c mice to P.falciparum CSP vaccine given by D-D and D-V regimens (Experiment 1) .
Regimen Dose (p.g) A20-CSP A20-CSIJ\M
VAX PBS Ratio (VAX/PBS) VAX PBS Ratio (VAX/PBS)
D-D 2 582 73 8 598 474 1.26
10 750 153 4.89 390 548 0.71
so 757 603 1.26 658 734 0.90
D-V 2 803 1026 0.78 1230 711 1.73
10 806 1098 0.73 728 730 1.00
50 1078 1317 0.82 642 1003 0.64
IFN-y EUSpot assays were carried out as described in Section 2. Freshly isolated spleen cells were pooled from 8 BALB/c mice per group 2 weeks after the last immunization
and incubated with A20 cells transfected with P. falciparum CSP, CSIJ\M, or emp[y plasmid as control. Data is presented as antigen-specific IFN--y spot-forming cells per
million spleen cells (SFC) after background control responses have been subtracted. The ratio of SFC of corresponding Vaxfectin~ and PBS groups were obtained and are
reported in the MRatio (VAX/PBS)" column.
obtained for A20-CSP vs. (A20-CSP + A20-SSP2 + A20-LSA 1 + A20- tion with 2 J.Lg unformulated pDNA CSLAM (Tables 3a and 3b, and
AMA 1 + A20-MSP1) were 582 vs. 602; 750 vs. 755; and 757 vs. 775 Fig. 2).
respectively.
In the Vaxfectin® formulated CSP at 2 J.Lg dose group given via 3.5. IFN-y ELISpot response to P. falciparum antigens by
the D-V regimen, we obtained robust responses against CSP due to multivalent CSLAM and 5 individual antigens
the CSP component from the NYVAC-Pfboost. However, expected
background responses against the other four CSLAM antigens were Data presented above showed that the immune enhancing effect
also noted due to the immunogenicity of the NYVAC-Pfl boost even ofVaxfectin® formulation was generally greatest at low dose pDNA.
in the absence of prior antigen-specific priming with the non-CSP Therefore, we next assessed the ability of Vaxfectin® formulation
antigens. Summed responses induced by NYVAC-Pfl when injected to enhance responses induced by low dose (2 J.Lg) immunization
alone against all 5 antigens was 95, which comprised of A20-CSP with each of the five CSLAM plasmids injected individually or as
(19)+A20-SSP2 (44)+A20-LSA1 (O)+A20-AMA1 (21)+A20-MSP1 the CSLAM mixture in Experiment 2; those studies used only two
( 11 ). priming doses of pDNA instead of the three doses given in previ
In other studies, we have reported observed suppressive effects ous experiments in order to better dissect any potential differences
with a 9-gene mixture which included the CSLAM antigens (23]. (Table 4 ). In almost all cases, Vaxfectin® formulation enhanced
The CSLAM mixture was specifically down-selected from that 9- responses against the respective APCs (Table 4).
gene mixture on the basis ofimproved compatibility with negligible Results from the experiments reported above suggested that
antigen interference [23]. the enhancing effects of Vaxfectin® formulation were more pro
nounced under suboptimal immunization conditions (namely, low
3.4. Effect ofVaxfectin® formulation with low dose pDNA on pDNA dose and fewer primes). Since heterologous D-V immu
antigen-specific IFN-y responses in D-D and D-V immunization nization regimens are more immunogenic than homologous D-D
regimens regimens, we next asked whether Vaxfectin® formulation could
enhance a suboptimal D-V regimen involving a single prime with
We have previously shown that Vaxfectin® formulation was an extremely low dose pDNA in Experiment 3. For those studies,
most effective in enhancing immune responses at low doses of mice were primed once with 0.4 J.Lg pDNA encoding CSP, SSP2, LSA 1,
pDNA (29 ). We hypothesized that immune responses obtained with AMA, MSP1, or CSLAM, and boosted 4 weeks later with NYVAC
low dose pDNA formulated with Vaxfectin® would be comparable Pfl. Results showed that mice were sufficiently primed by the
to responses induced by higher doses of unformulated pDNA.Initial Vaxfectin® formulated low dose pDNA for boosting by NYVAC-Pfl
studies with 2, 10, and 50 fJ.g pDNA in PBS, administered via a D-D in this abbreviated immunization regimen. Enhanced responses
regimen, showed a general dose response (Tables 3a and 3b, and after priming with individual antigens were detected against all
Fig. 2). As hypothesized, Vaxfectin® formulation oflow dose (2 J.Lg) tested APCs, reaching statistical significance with almost all APCs
pDNA CSP and CSLAM vaccines administered via a D-D regimen (Table 5). Furthermore, as seen in the earlier experiments, no
resulted in high levels of response against A20-CSP and A20-CSLAM evidence of suppression in the mixture was noted taking into con
APCs that were comparable to or better than those achieved by sideration that the amount of CSP pDNA was one-fifth the dose
unformulated high dose ( 10 and 50 J.Lg) pDNA (Tables 3a and 3b, of the univalent vaccine being tested. Another group of mice that
and Fig. 2). A similar trend was noted for the D-V immunization received the NYV AC-Pfl boost but no pDNA prime showed minimal
regimen, although the responses were more variable. responses (data not shown).
Our third hypothesis was that a D-D immunization regi
men with low dose pDNA formulated with Vaxfectin® would 4. Discussion
yield enhanced immune responses comparable to those obtained
with unformulated low dose pDNA administered in a heterolo Because of the complexity of the Plasmodium parasite life cycle,
gous DNA prime/viral boost (D-V) immunization regimen. Data many believe that an effective subunit malaria vaccine will need to
showed that D-D immunization of Vaxfectin® formulated low contain antigenic components from more than one developmen
dose (2 J.Lg) pDNA CSLAM vaccine induced high responses against tal stage. Multi-antigen vaccines against malaria given as plasmids
A20-CSP APCs, which were comparable to the responses induced or in the form of recombinant mastocytoma-transfected cells have
by D-V immunization of unformulated low dose (2 J.Lg) pDNA successfully protected mice (9.46) and monkeys [47.48) against
CSLAM vaccine (Tables 3a and 3b). However, for the non malaria, establishing the feasibility of a multivalent malaria vac
CSP APCs, Vaxfectin® formulation of low dose (2 J.Lg) pDNA cine. Ease of combining pDNA vaccines, as compared to other
enhanced D-D induced immune responses; however, levels conventional methods such as recombinant proteins and viral vec
approached but did not reach those obtained by D-V immuniza- tors, has made DNA an attractive platform for the delivery of
Table3b
IFN-"'f ELISpot response in BALB/c mice to P.faldparum CSLAM vaccine given by D-D and D-V regimens (Experiment 1) .
Regimen (D!Logs)e A20-CSP A20-SSP2 A20-LSA1 A20-AMA1 A20-MSP1 A20-CSLAM Summed CSLAM
D-D s1o20 2V755A139X 2P1454B143S 005(RV. 32aA11tiX o / PBS) V463A502X588 P832B71S138 4 00(3RV.. 85aA36tiX o / PBS) V355A297X873 P753B987S184 02(1RV.. 40aA11tiX o/ PBS) 11V1040A446X799 P59B331S51 96 (131RV.. a95At87iXo / PBS) V355A488X134 P4B26512S114 (013RV.. a61A2t9Xio / PBS) 1V11776A276X498 1P115B93S223 646 0(1R1V.. a91A23tiXo / PBS} 223V561A463X635 23P112B29S803 86 30(1RV.. 8a1A1t6Xi o / PBS)
D-V s1o20 666345873 321559602 321..367119 111321666850 11821075857 111...050770 111574818430 111791363433 001...957115 111748070768 111621287487 011...944124 111634899625 11655952340 201...119144 222102617868 222224476338 001...980800 666748592155 646501155680 111...006468
fTIaFhlNde-p "r'aaf rtEuioLm IoS Cfp SoSFtP C,a S sosSfaP cy2os, rLwrSeeAsr p1eo, AcnaMdrinrAieg td .V MaoxSufPte 1ca.ts C idnSe~L sAacnMridb. oePdrB eSim ng rpSoteuycp ptsiola nws em2r. iedF oraebss tcahoilnyn etisrdoo all.an Dtdea data rse ips r leepeprenos rceteendltles i dnw atesh reae n "pRtiogaoetlineo-d {s VpfrAeocXmi/fPi c8B IFBSNAt -Lc'YBo l/sucp mmonti-c.f eo rpmeirn ggr coeulpls 2p ewr emekilsli oanft espr letheen lcaesltls i m(SmFCu)n aizftaetri obna cakngdr oiunncudb caotendtr owl irtehs pAo2n0s ecse lhlsa vtera bnesefenc steudb twraictthe dP..
Table4
IFN-"'f ELISpot response in BALB/c mice to P.faldparum individual and CSLAM vacdnes given by D-D and D-V regimens (Experiment 2).
Regimen Vaccine Individual and CSLAM vaccines vs. individual A20 targets
Summed CSLAM response
A20-CSP A20-SSP2 A20-LSA1 A20-AMA1 A20-MSP1 VAX PBS Ratio
VAX PBS (RVaAtiXo/ PBS) VAX PBS (RVaAtXio/ PBS) VAX PBS (RVaAtXio/ PBS) VAX PBS (RVaAtiXo/ PBS) VAX PBS (RVaAtXio/ PBS) (VAX/PBS)
D-D CInSdLiAviMd ual 429200 15577 33..19 2269230 456220 41..43 1509077 474873 11..33 11282238 11118630 11..15 1861470 1305337 21..46 4,277 3160 1.4
D-V CInSdLiAviMd ual 1267630 239727 40..49 31927370 3888480 11..40 24842733 31638120 21..22 42086670 21357938 11..87 23859470 21068080 11..96 11,087 6178 1.8
Regimen Individual and CSLAM vaccines vs. A20-CSLAM targets
CSP SSP2 LSA1 AMA1 MSP1 CSLAM
VAX PBS (RVaAtiXo/ PBS) VAX PBS (RVaAtiXo/ PBS) VAX PBS (RVaAtXio/ PBS) VAX PBS (RVaAtXio/ PBS) VAX PBS (RVaAtXio/ PBS) VAX PBS (RVaAtXio/ PBS)
DD--VD 2254170 618633 33..32 23.483233 3475487 51..30 41404870 4177470 11..15 31949032 2176833 11..88 2569437 1914470 41..43 42646333 23316480 11..14
AIrFe2Ns0p-' oYcn eEsllesLs ISt rhpaaonvts eaf esbsceateeynds swwueibtrhter a Pcc.a tferardlide. dpT ahoreuu trm aa tsiC odS ePo.fs SScFrSiCbPe 2o.d fL ciSnoA rS 1ree. AsctpMiooAnn d12.i. n MFgrS eVPasxth.f lyCe cSistLoiAnlaM~t e.a don rds epPmlBeSep ntgy rc opeullalpsss mw weider erae sp oocbootlnaetidnro eflrd.o Daman td8a aBisrA epL rrBee/psceo mnrttieecdde iapnse trah gnert io"gRuepant -2ios pw(eVecAeikfXisc/ Pa1FfBtNes-rr'Y tc hsoeplu olmat-snfto .i rmmminugn cizeallsti opne r( imndililvioidnu sapl laenetnig ceenllss o(Sr FCCS) LaAfMte)r, abnadck ignrcouubnadt ecdo nwtirtohl
M. Sedegah et al./ Vacdne 28 (2010) 3055-3065 3063
~ .gVra~:a.i' .gVar~:a.i ' aen .~B,2 !§o;,.9~ ':2! pmraimlaeri-ab ovoasctc iinmems. uOnuizra ptiroenv iroeugsim deantas , isnudcicha ates dp rtihmaitn ghe wteirtohl opgDoNuAs
~c. 0,1: >...... Ill 0,1: >...... C-"ta a. ~cc2 ~!.l.. icIl!! hanodm oblooogsotuinsg i mwmituhn rizeactoiomnb riengainmt evnirsu [s4,1 a.4re9 ).m Tohree c oefmficpalecxio luosg isthtiacns
t~j Var:a.I M.Ma.n. ·cCol l Var:a.I 1""1 s":, E"ot~CU:ll e.£r.a. caonud lcdo bstes aasvsooidceiadte idf wa istuhi tmabalneu fmaecatunrsin ogf a e snehcaonncdi nvgac tchinee i mplamtufonrom-
'eCall ~u t0 j:~;·a~ genicity ofpDNA vaccines could be identified.
V:eII § c1.0..n00. ~== § 0a n "cB '~'i,ui c oec. antWigeen hica vceo mprpeovnioeunstsly oref pao 5r-teadn dre 9d-ugceende i mmmixutunree r aess pcoonmsepsa rteod t hteo
~ ·~~~ the responses obtained when given as individual plasmids [23,50).
.Z!2i .V~>ar..:.a.i... ' . 1I"""1: caCID .i ~cc.0t..:0.i. I.~E~i Ntw} Q~0I . ·:·:-~g:c:r6S::- . c =e.8..-!~.2.c.'. O '·~~>~ :00:: : mmtmSauairixxgbtettseuusetrr qeerheusa,s e vdponeeoft s nstphishglaeionsssw a dmwtneoi iddwnt honeCo -laSisumdeLtvAl iesneMecars,rtt eieiotod huen asCf sftSes twuucLAtpdaspiMs e dr secum asleespi xdiatvot beuu l asere en tfoo ftiei ngfic de itnnsenod.n cnuStohictfumiuyndm abgie i a5nsmn- agw utepiilonrttihnie--
V~a:I Var:a.I C0D0 a0Nn0 ·uCcol l Var:aI. .e..n.. .·:E§~I !a..§_g~~O " £~c1'1:1 e[5ff1eH)c. et roefi nd,o swee a nhda vpela sumseidd ftohrims u5la-gtieonne fomr ioxptutirme atlo m audltdi-reasnst igtehne
~ § aN0n 00v 0 =e~=; >~ .va.n. . .~t!C!l!;l .8"~!';.. .:.E02: iimmmmuunnoizgaetnioicni tyre. gWimee nssh, ofwor mthualta, tiwonit ho fh loomwo dloogsoeu sp laDsNmAid (Dw-iDth)
e·;;cCcCe:::ll:;ll. .~g~Vra~:ai.' .0}- a~.0.D..0.. .0.0. :a00<ND= 0. ! .~g~Var~:ai.' .C.D. :a~..a.... 0~~NrCr0 ll ~ "c~~~!·'u; ~c>f:~: WfbiVnolaedremx u tffoceuue clrtadhttih ntoibe®soyern es oDwnhbh-oiDttaawh nin icVamee agdsmxe ifwumneneicmtitrhzianu alh® nttiir egoweh nrnaee dssrr ep dhwgooihgnismseheeere epsnr,Db s ciy Nonw mdAthiu ptewhca ii irntmaehg dmo ll oeuwuwvtnie tf eohlds ro rumcesonesumfp olpoa prDntmaisoNreuanAs-- .
<zQ·~cCe0c>~ ll . ~~=<= §Var:aI. Vra:ai.' .aN10.."n.' 1. Nv a.N;....n. .. c...n.. i·,uc>oCl l §VreI ~aeCnnD .;~.eI~C~:rc~D!_lI;!lc 5 e;,c.·-~Ic:C:r~!cQCa -l!l.a c: --I.,,II£giC~5a.!! ! l!ll d(fmhpIoaDiDod rat-emmN VndAnou ) v plfo oaarDtiegcc nNgotcf uaiiuAnmracsee, ie I l.evhi natTavae.n hcettdeecTilss ir hn aodwtiesldrio aa dmpgsni soln isa nnuoftaieosfst o c ttrs reettDimihaorgN ecnn thAi h siff a ii[trcm3hnpsa0o otrn] sair.dmte e eh Aefimcenin-notdro edesunreticcon saoeelgtorfmrd flagie sebowtciirnioutn iictsnvsahe e tn tmu uhtatd hnuanyvefltd ot i iVr hrpaeumaalsale sxeus f bilepeasoocrlutoa ftegti stnoda--t
Q0~ . .~g~~ Ioq";' 'Q~D. -0}a ~a. Var:ai.' 1""1 <~~ c=~5· ;~..;. g[4e0s)t.e d that the enhanced antibody responses are IL-6 dependent
·..8'~sc>CICQ>~a2Soll l.l . ~0<~ §Vcr:aI. :v11e:""n;:''11 8a1~"n{'1 ·u~cCol l !.~f g~~ e0vNonq0 ; Q~I . .'.-·:·r_!:egi,;-a; :6i: gaccu:~e,,"2uo ~.c'V r -.oeu~~I:oaI3!I:! stptimeehrcneomtIctwit oulw iesnnndhii otc iohngatuhd eldl uadmnact it boceaVedid ta pey nxbl rsof(eye.bt vcePoHi.tdo tiofnh uawt® lshTce laiy vpfct oea getrrrehlm,lu netmaueh nrl ereaadc attor ieeadondsonns uutt irilnotbtu s fooc t rhtldfseo eyt phwc oerPa e.r d nstcyoenpuosdooree trnl h eibsPien eeyrmt sCe e)sS o vatPsduari eedlnup lic cealeawost esn hpdmwsir ciaoisidhns--
·';'uI~:':,a=a;,;u>uc1I;I;1: ) 1 ;~0:t~;g ;l Var~.:a.I g~Var~:ai.' :.~.8...c ~c.t.:.:..i .NM-0...} .a ~00I. 0 ~ ~< .~§g~ ia~a. .C0aJ-n0l a~I . ~.-·'~g_I"a~~=u;!c!2.' a :" s<~:<.=l:E.0=:'-·.=.· j ~-ec.~Sec0~1c,5c1 a .r0 ~ aci>~-.• tb5tamDhhsr-Neeogi cp aeOAemrmnd ,uo e viarutxa a esltdnC ctcuciactStvoriitibevnLaamoAee leipdsf Mnuena y fretrt ftvh eainhcvaednheaac dacratcc o nycipTvn c iPpnaea ecaelB gdied r[aS e2lCaliT -nn3tSfr eso,te5Lc t ras Ae0moPbplM)ulsuo.l a ernlaaOs nvspnmtcaeevdreocseed d crv aoaapiiinuonflDglem ,utaa Ni sb niocnAs otaudsip gdn[troo2 ey werd9 nioanar n )zec.dit -nohassut pi ecteceoelereon fs ecmcstrahretoeipbasobnoll nliuecsna shenseot .gn fwteTa tht nhheaoiedenltfl
ra·<_.~~·:B:uCusietEaca-:.-l !l l . ;l'~·.:c·'';a~~uc.:o>I:5aaj;;.I; I; : <0VVN~ II ~a.~>~. g ~Vcr~:a.i' :NN.~N. c. ~0Nt ect-aNvwnrn}. a 0.o.I..q ..; ·~~~s,=u>~oc!~C1!= ! !l! l 1 ~~',8c>C l l .~§a~2.g : Var~:.a.i' I~NNMIC"aD '' vQ~. -.~~·-::·~!to:rt~!::;:c--a:;8:ca l N.ia-..-V~~.t~ilEcj@~::bl!I:al o =·.):''~'~c'"§QD.0,l-.'~.• ! -! a O'.8:.N"6"aC~~!ct502Q!'l'l ' aaeupcmntaus aonripm orfolcniep cnctoisvsaeoxkaadi rmistennceiuntnytcep-nro gs1ienadtnt 7 eaiart eim ofe5bdpsfwdef s mic (ire[thtE5aewtunheo2lnBcn o r )mceeAdi. mze ,pa-eo Oe 1otlrfafadi7otnon as h 5zkrw gnme )eoaet ribiiyintan gdthossetnee i tt gDdruanhaiae md Ni nmctmnieatAme ic-idgne ssxou evtr ptimionaiunbnenczi-ro pcz 1ceoAidmeei f inoioytd(tcieeiAtc f t us i eaPMwEoss n.Lc nuiAfhdaItmar Sahw fn1laAvco c) ice.i ncet epe hetoaklaid lr tcenurpeyephyuflltremr aashmsoro rsro t womesfwDnr ycihetsanNihnydsets-Aeret p1r Dea e ro nvt gNin be(onainsMAsidctdnoeet c iiSdms dctvinsh,iP iniieodagixlignssr)-t--
.55I ·'::a;;I: ~a. tj :Qv .~l 'aJ~~ additive effects on protection against sporozoite challenge [53). The
~~11)1~~&V:0c:~c0c:1I; . . ·~:.cuCoa5l l 0~ >~ :.;·'~a::5a;s;n;I;: s~:~aEn :;.·'':,:csa;5a;;I;: l·a,uc>oCj. l l §~a. 0.a.1.."n..0.'.1. ~~:l:;~:~~:::&;- . l~!:·.0~~~!ci !o g ·:8I~g~:~~!:!: -~:~··' ! fusa=!:c!;; , uomhsdrinaunaouevptiravle iptsa ti oyvptltaeoa rrgoetn l eeosfat e tnfwvihn ntatiha tscveteihc atdm ieccnc c ouhelpiinnesnlat.cerii rc-eesata ,aps pltrei tr goetsoee pfvsl tmeii.tfd icenmeui agc lutaly ilylvwdct laideti-htlii,ehtm oiwon smcntoe hva uuatallan ldcleree cgxbn iernpeget aeeesmtrd,pii oom oiantrnee g.si ns aWep ti nareahoslxpi ttlpeep emevcr octitdhoitaveeerceedn hs ctt utehhwhpa aaieinnln-tl